 Figure 1: Ancel Keys (January 26, 1904 – November 20, 2004) was the American physiologist and epidemiology of cardiovascular disease (CVD). He was responsible for two famous diets: K-rations formulated as balanced meals with a long shelf life for combat soldiers in World War II and the Mediterranean (Cretan) diet. Keys is shown (at right, above) two months before his 101st birthday.
Figure 1: Ancel Keys (January 26, 1904 – November 20, 2004) was the American physiologist and epidemiology of cardiovascular disease (CVD). He was responsible for two famous diets: K-rations formulated as balanced meals with a long shelf life for combat soldiers in World War II and the Mediterranean (Cretan) diet. Keys is shown (at right, above) two months before his 101st birthday.
The Seven Countries and Adventist Health Studies
Nathan Pritikin drew his initial conclusions about the effect of dietary change from classified data he was privy to during World War II (WWII) on the patterns of age-associated disease in Europe as a consequence wartime calorie restriction and severe reduction of fat intake due to the severely reduced availability of meat and dairy products. He also observed that the incidence of age-associated degenerative diseases was very low in human populations where the diet was very low in fat (~10% of the total caloric intake), contained no refined sugars and consisted mostly of fresh vegetables and fruits with very little meat being consumed. Similarly, the physiologist Ancel Keys (Figure 1), who was working with the Army Quartermaster Corps in developing K-Rations,[1] became involved in the Army’s program to create scientifically informed re-feeding programs for POWs and civilians suffering from starvation, saw the same kind of data. Unlike Pritikin, Keys had the opportunity to do human experimentation afforded by wartime conditions.[2, 3] Keys working hypothesis was different than Pritkin’s, namely that it was primarily saturated fats in the diet that were responsible for the high incidence of cardiovascular disease (CVD) in the affluent and well-fed West.
This epidemiological approach to identifying patterns of food consumption associated with increased or reduced risk of degenerative disease was also being pursued around this time in the US, by physicians at the Loma Linda Medical Center in Loma Linda, CA. Loma Linda was an almost exclusively Seventh Day Adventist community at that time, and these physicians believed that their patients, who practiced a vegetarian diet in conjunction with abstention from tobacco and alcohol, were considerably healthier than the non-Adventist population in California. They began a study of diet, lifestyle and the incidence of disease and all-cause mortality in 1958; the Adventist Health Study-1 (AHS-1) [4-26]
Keys returned to Europe after the war and began a study of six European countries, which later became the Seven Countries Study. [27-72] The dietary recommendations which emerged from the Seven Countries Study are commonly referred to as the “Mediterranean diet.” However, the use of the words “Mediterranean diet” to describe these recommendations is a misnomer. The countries of the Mediterranean basin have large differences in diet, lifestyle and in their corresponding rates of morbidly and mortality. The country with the lowest death rate (14.0 – 18.0 per 1000 persons), is Crete, whose death rate has been at this level since at least 1930. [73] The diet of Crete is archetypical of the ‘traditional’ Greek diet before the introduction of continental European and American foods into Greece after ~ 1960.
The Seven Countries Study was the first to generate robust data on the incidence of cardiovascular disease in a range of populations (US, Finland, The Netherlands, Italy, the former Yugoslavia, Japan and Greece) with a fairly broad spectrum of dietary patterns. The study showed differences on the order of 5 to 10-fold in coronary artery disease (CAD) between the populations studied. [36, 50, 74]
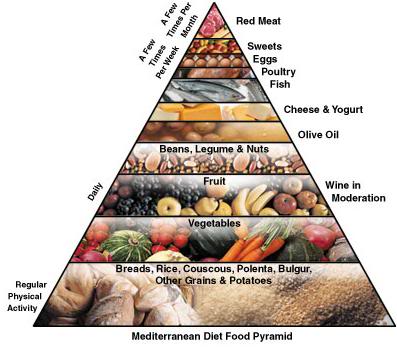 Figure 2: The Cretan diet food pyramid.
Figure 2: The Cretan diet food pyramid.
Both the Seven Countries Study and the AHS-1 demonstrated large reductions in disease-specific morbidly and mortality, as well as in all-cause mortality; primarily as a result of diet and lifestyle differences. In the case of the Seven Countries Study, extensive follow-on research using well designed prospective studies, resulted in the development of a set of dietary guidelines which became known as the “Mediterranean” or more correctly, the “Cretan diet.” The guidelines which constitute the Cretan diet satisfy Level-1 EBM criteria for extension of the mean lifespan by ~10 years, reduction in all cause mortality, high levels of compliance, and very importantly, titratability. In other words, the degree of compliance with the diet is roughly commensurate with the benefit that results. [75-77]
What About Cholesterol?
For thirty years an acrimonious debate has raged in the scientific and medical communities over whether cholesterol, or any molecular species of lipoprotein, “causes heart disease” or other CVD. The causes of the inflammatory events that underlie the start of arterial plaque formations are complex, possibly multifactorial (e.g., genetic, viral, microbial, environmental, etc.) and by no means fully elucidated. Keys, Pritikin and many others mistakenly believed that “elevated” serum cholesterol was the primary cause of CVD. This hypothesis is well supported by the epidemiological data. However, there are many people who have normal or even slightly low levels of total cholesterol, or of the low density lipoprotein (LDL) species whose oxidation is usually cited as the motor of atherogenesis.
 However, the observations of Keys and Pritkin extended beyond a cause and effect relationship between cholesterol and CVD. In different ways, both men demonstrated that altering the total serum cholesterol level and/or the ratio between the LDL and high density lipoprotein (HDL) species, they could reduce the incidence of the disease. In Pritkin’s case, he even demonstrated that the disease could be reversed by the expedient of a very low fat diet.[78-80] Pritkin demonstrated his theory on a very small population of people; principally those who read his book, or otherwise followed his dietary advice. Keys, on the other hand, conducted an experiment on a grand scale.
However, the observations of Keys and Pritkin extended beyond a cause and effect relationship between cholesterol and CVD. In different ways, both men demonstrated that altering the total serum cholesterol level and/or the ratio between the LDL and high density lipoprotein (HDL) species, they could reduce the incidence of the disease. In Pritkin’s case, he even demonstrated that the disease could be reversed by the expedient of a very low fat diet.[78-80] Pritkin demonstrated his theory on a very small population of people; principally those who read his book, or otherwise followed his dietary advice. Keys, on the other hand, conducted an experiment on a grand scale.
Throughout the 1960s Keys campaigned relentlessly to persuade physicians, public health authorities and the public themselves (directly) to replace the bulk of the calories they consumed in (saturated) fat with polyunsaturated fat. The purpose of this international dietary intervention was to reduce the serum cholesterol of the population, and thus the incidence of CVD. This effort enjoyed unprecedented success and it has resulted a doubling of the proportion of the unsaturated fatty acid, linoleic acid, in the tissues of Americans between 1960 and 1975.[81] The mortality rate from coronary heart disease (CAD) in the US began to fall, starting in 1968, and it has continued to decline since then. It has been estimated that approximately 50% of the decline in CVD is as a result of dietary and lifestyle changes, exclusive of the reduction in tobacco abuse.[82]
As a scientist, I am acutely interested to understand the details of the pathophysiology of atherosclerosis. As someone who wants to avoid CVD, I am much more concerned with what works, even if the biomechanics are incomplete, or even contradictory. In this case, what works is that on a population basis, blood lipids are highly predictive of the risk of disease. Similarly, for most patients, raising HDL and lowering LDL are protective against both the onset of CVD, and to a lesser extent, its progression. Lipid status is thus a useful screening tool, as well as an instructive guide to the individual patient’s likely response to treatment. It is not necessary to believe that “cholesterol,” or any particular species of lipoprotein “causes” CVD. It is only necessary to understand that they are, at the least, useful biomarkers on a population wide basis and that they are often useful in the intelligent management of individual patients.
Table 1: Fatty Acids Ratios in Different Diets
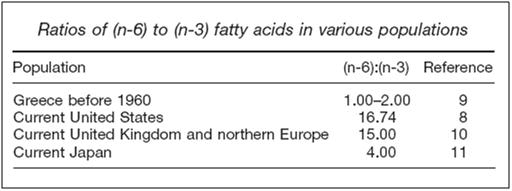
From the inception of the AHS-1 in 1958, and the Six Countries Study around the same time,[68, 83] the primary focus of the research, as was the case with Pritikin, was on the possible relationship of the diet to the etiology of CVD, with special emphasis on the fatty acid composition of the diet. The 5-year follow-up in the Seven Countries Study found favorable all-cause death rates in Greece, Italy and Japan, as compared with the other countries, including dramatically lower rates of CVD in Crete, and to a lesser extent in Japan.[84]The diet of Crete has in common with the diet of hunter-gatherers similar quantities of antioxidants, saturated fat, fiber, monounsaturated fat and, very importantly, the ratio of (n-6) to (n-3) fatty acids. [75-77]
One the basis of insights gained from the Seven Countries Study a wide range of epidemiologic investigations, controlled clinical trials and relevant animal experiments have confirmed the hypotriglyceridemic, anti-inflammatory and antithrombotic aspects of (n-3) fatty acids (28 –35) as well as the criticality of (n-3) fatty acids, particularly Docosahexaenoic (n-3) (DHA) acid in the diet for the normal development of the retina and brain in the human infant. As a consequence of these insights, a study of the (n-3) fatty acid composition of diets that were known to be associated with reduced rates of CVD and cancer was undertaken.[75] The initial conclusion was that the high olive oil intake, which accounts for ~35% of the calories consumed in the Cretan diet, was likely responsible for the low rates of CVD and cancer. However, the Japanese have a similarly low incidence of these diseases and yet only ~11% of their calorie intake is from fat, none of which is from olive oil.
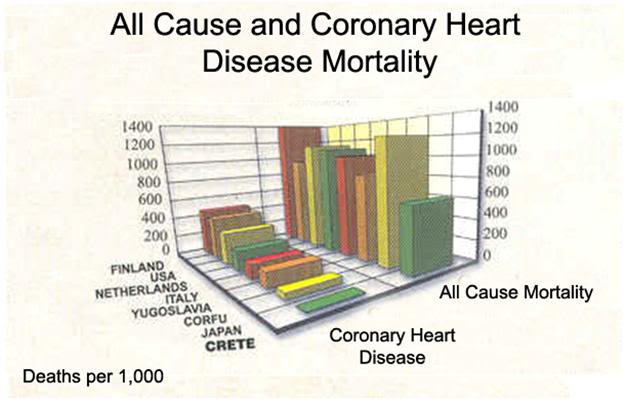
Figure 3: Mortality and morbidity difference between populations of patients with coronary artery disease (CAD) eating the Cretan diet and those on the low fat American Heart Association Step 1 diet[151]
The common factors between the populations of Crete and Japan were that they both consumed large amounts of vegetables (including wild plants), fruits, nuts and legumes, all of which are rich sources of folic acid, glutathione and vitamins E, C and other antioxidants. Wild plants are rich sources of (n-3) fatty acids and antioxidants and their consumption is not confined to humans. Both in Crete and in rural Japan, chickens and other livestock, such as goats and cows, are free ranging and consume wild vegetation in abundance (and in the case of chickens, insects, arachnids and worms) which are rich in (n-3) fatty acids and antioxidants, as well as cytoprotective and vasculoprotective trace minerals which are concentrated in the food chain. The result is poultry, eggs and milk which contain radically different ratios of (n-3) to (n-6) fatty acids and are enriched with selenium. For example, eggs from Crete have a ratio of (n-6) to (n-3) of 1:3, whereas the US ‘battery egg’ has a ratio of 19:4. Of course, the presence of this favorable ratio of lipids in eggs is also reflected in prepared foods which contain eggs (such as noodles, some breads and soups, etc.) and in the flesh of the animals that are slaughtered and eaten.
Analysis of the serum (n-3) fatty acid levels of the populations in Crete and Japan demonstrated that they had had higher concentrations of (n-3) fatty acids than did the other populations in the study, all of whom had a far high incidences of age associated degenerative diseases. The two populations with the lowest CAD in the Seven Countries Study consumed the highest amount of α-linoleic acid (α-LNA) the major sources of which were the wild herb purslane, walnuts and figs. By contrast, the Japanese obtained their α-LNA from canola and soybean oils. Interestingly, the Seventh Day Adventists, who experience an increase in mean lifespan of 7.28 years (95% confidence interval, 6.59-7.97 years) in men and by 4.42 years (95% confidence interval, 3.96-4.88 years) in women over that of their non-Adventist cohorts in the US[10], consume a vegetarian diet that is rich in nuts and oils containing α-LNA. The SDAs, like the people of Crete, have not only higher levels of α-LNA, but also lower levels of linoleic acid[9, 85, 86]
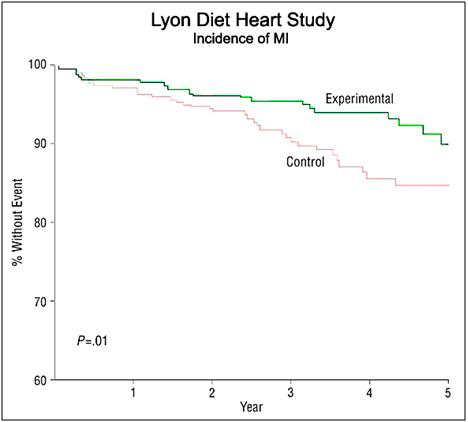
Figure 4: The Lyon Diet Heart Study demonstrated a 50 to 70% reduction of the risk of recurrence of myocardial infarction (MI) after four years of follow-up in coronary heart disease (CHD patients. The Lyon diet employed α-LNA as 0.6 to 1% of total daily energy or about 2 g per day in patients who follow a traditional Mediterranean diet. Supplementation with very long chain omega-3 fatty acids (c.1g per day) in patients following a Mediterranean type of diet was shown to decrease the risk of cardiac death by 30% and of sudden cardiac death by 45% in the GISSI trial. [87]
In 1994 de Lorgeril and Renaud published the results of a prospective study to evaluate a diet which contained the types and ratios of essential fatty acids (EFAs) found to be effective at reducing CVD in the Seven Countries Studies. The Lyon Heart Study (LHS), as it came to be known, was a randomized, single-blind secondary prevention trial that combined a modified Cretan diet enriched with α-LNA with that of the Step I American Heart Association diet.[87] The LHS demonstrated a reduction in all-cause mortality of 70% in the experimental population which consumed a diet low in butter and processed meats, but high in fish, nuts, fruits and vegetables (Figure 5).[87] The LHS followed subjects for 5 years after the start of the intervention and examined the reduction of risk for coronary artery disease (CAD) as well as in mortality from all cancers. The reduction in CAD was 56% (P 5 0.03) over that of control subjects, and in cancer mortality it was 61% (P 5 0.05)!
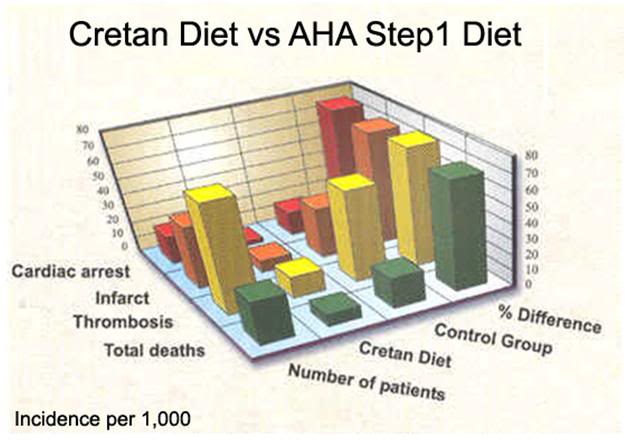
Figure 5: Cardiac morbidity and mortality in the Lyon Diet Heart Study. Of particular importance to cryonicists is the reduction in mortality from sudden cardiac death (SCD). [87]
Olive Oil, or Something Else?
 Figure 6: The author has serious questions about whether experiments conducted using industrially prepared laboratory animal chows (right) are representative of the results obtained when fresh fruits and vegetable as well as foods consumed in their native state are used (left).
Figure 6: The author has serious questions about whether experiments conducted using industrially prepared laboratory animal chows (right) are representative of the results obtained when fresh fruits and vegetable as well as foods consumed in their native state are used (left).
An initial and obvious conclusion from The Seven Countries Study was that a significant part of the reduction in morbidity and the extension of lifespan due to the Cretan diet was a consequence of the consumption of a large fraction of the calories in the diet in the form of monounsaturated fats (MUFAs), principally as a result of olive oil consumption. However, recent animal studies have yielded paradoxical results. For instance, experiments in green monkeys have shown that a diet high in MUFAs (olive oil source) causes atherosclerosis equivalent to that observed in animals fed a diet high in saturated fats (SFAs).[88] This effect appears to result from an increased secretion of cholesteryl oleate enriched lipoproteins, as well as due to an increase in the circulating blood levels of chylomicron remnants, which are highly atherogenic lipoproteins. [89-91]
These paradoxical animal results have raised questions amongst epidemiologists and nutritionists about whether MUFAs really have beneficial effects in humans. Green monkeys are metabolically and genetically different than humans and the human data indicate that dietary MUFAs have favorable effects on CHD risk. There is also a significant amount of mechanistic data that indicate that there are molecular species in olive oil that have potent antioxidant and anti-inflammatory properties. In particular, the polyphenolic compounds hydroxytyrosol and oleuropein have been shown to possess these properties both in vitro and in vivo.[92-96] There is also the issue of the way in which olive oil is incorporated into the chow for experimental animals (Figure 6). Olive oil incorporated into a manufactured chow along with other dietary ingredients is not the way in which humans consume it, and this factor should be taken into consideration in future studies.
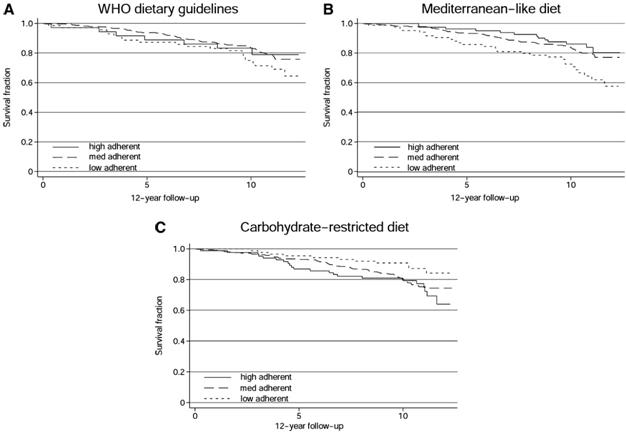
Figure 7: The titratability of the beneficial effects of the Cretan diet are nicely illustrated in this series of graphs showing all cause mortality over a ten year period with three variations of the Cretan diet; the world Health Organization recommended diet base on the Seven Countries Study, a broadly similar diet, and a carbohydrate restricted version of the Cretan Diet. Kaplan-Meier survival curves for individuals considered adequate reporters of dietary intake, grouped as low-, medium-, or high-adherent individuals to the dietary patterns investigated. Crude hazard ratios (HRs) and 95% CIs were calculated from Cox proportional hazards regression analyses with the use of low-adherent individuals as the reference group for each dietary pattern. A: World Health Organization (WHO) dietary guidelines, according to the Healthy Diet Indicator: medium adherent (HR: 0.70; 95% CI: 0.43, 1.15), high adherent (HR: 0.97; 95% CI: 0.45, 2.07). B: Mediterranean-like diet, according to the Mediterranean Diet Score: medium adherent (HR: 0.68; 95% CI: 0.44, 1.04), high adherent (HR: 0.29; 95% CI: 0.12, 0.70. C: Carbohydrate-restricted (CR) diet, according to the CR diet score: medium adherent (HR: 1.92; 95% CI: 1.02, 3.62), high adherent (HR: 2.17; 95% CI: 1.05, 4.45).[148]
Anti-inflammatory and Cytoregulatory Lipids in the Cretan Diet
One proposed resolution to the paradoxical animal findings regarding the atherogenicity of olive oil in the laboratory is the observation that both the high fat MFA diet of the Cretans, and the low fat PFA diet of the Japanese, are rich in (n-3) fatty acids and antioxidants, in particular resveratrol, glutathione, vitamin C, vitamin E, lycopene, b-carotene, polyphenols and polyamines obtained from fruits, vegetables, wild plants, and olive oil. [97] [98-101] Additionally, both diets are enriched in α-LNA and eicosapentaenoic acid [EPA, 20:5 (n-3)] from the consumption of large amounts of fish, relative to the control countries.[159, 171, 174] Because olive oil is high in the monounsaturated fatty acid oleic acid [18:1, (n-9)] and low in saturated (n-6) fatty acids it cannot compete with the endogenous desaturation and elongation of α-LNA, or with the incorporation of α-LNA into the constituent phospholipids of cell membranes. This is particularly important in the case of red blood cell (RBC) and platelet membranes, where they act to increase the deformability of RBCs and decrease the aggreability and adhesions of platelets. [102-108]
The ratio of (n-6) to (n-3) lipids in the Cretan diet is between 2:1 and 1:1, which is very close to the dietary ratio of the Japanese, as that of hunter-gatherer societies. The beneficial effects of such a ratio and their importance in normal growth and development [109, 110] as well as in the reduction of risk for CVD, hypertension, type II diabetes, osteoarthritis and, to a lesser extent cancer, are voluminously documented in the literature.[111] [112-116] The traditional Greek diet is very low in animal fat and thus the saturated fat content is quite low (7–8%). This low intake of SFAs is complemented by the high intake of (n-6) and (n-3) EFAs which are also rich in phytoestrogens and other phytochemicals as well α-LNA, vitamin C, vitamin E and glutathione.[117, 118] These molecules have been shown to have hypoglycemic, hypocholesterolemic and antitumor properties in animal experiments.[119-124] Consistent with these findings is the fact that the mortality from breast, prostate, bladder and colorectal cancer is lower in both the Cretan and the AHS populations than is the case for controls. [14, 20, 86, 125-129]
The principal EFA in the US diet is LA, an (n-6) fatty acid which is the precursor to the eicosanoids – molecules which have proinflammatory and cytoproliferative effects. The EFAs are converted to prostaglandins by the cyclooxygenases and to leukotrienes (LT) by the lipoxygenases. Arachidonicacid [(AA); 20:4(n-6)] and EPA, an (n-3) fatty acid, compete for cyclooxygenases and lipoxygenases, resulting in the production of eicosanoids with opposing effects. In general, AA-derived eicosanoids, such as the 2-series prostanoids and 4-series LTs, have pro-inflammatory effects, whereas EPA-derived eicosanoids, such as the 3-series prostanoids and5-series LTs, have anti-inflammatory effects. A focus of recent research has been to understand the importance of the (n-6) to (n-3) ratio, rather than the absolute level of either species of PUFA in cancer prevention.[102, 130]
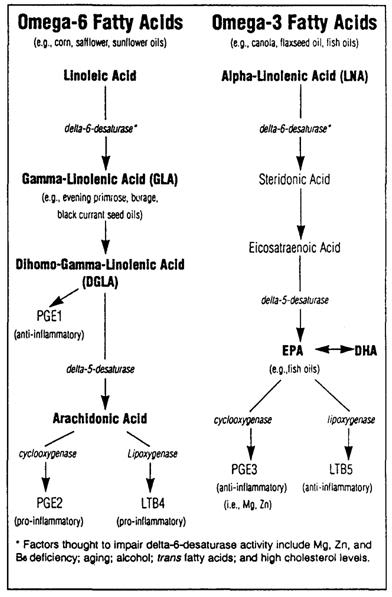 Figure 8: The major active product of the omega-6 fatty acids is arachadonic acid which is converted to the 2-series prostaglandins and 4-series leukotrienes by the action of cyclooxygenase. The 2-series prostaglandins are pro-inflammatory. In addition to the AA produced endogenously there are vast supplies available from the diet, most notably in meat, eggs and peanut oil. In the Western diet there are comparatively few products of omega-3 metabolism to moderate the pro-inflammatory action of excessive dietary omega-6consumption. If the amount of omega-3 fatty acids in the diet is increased, their metabolites (primarily EPA and DHA) compete with arachidonic acid for access to cyclooxygenase resulting increased production of anti-inflammatory mediators as well as a decrease in the pro-inflammatory mediators, thereby significantly reducing the ratio of pro-inflammatory to anti-inflammatory mediators.
Figure 8: The major active product of the omega-6 fatty acids is arachadonic acid which is converted to the 2-series prostaglandins and 4-series leukotrienes by the action of cyclooxygenase. The 2-series prostaglandins are pro-inflammatory. In addition to the AA produced endogenously there are vast supplies available from the diet, most notably in meat, eggs and peanut oil. In the Western diet there are comparatively few products of omega-3 metabolism to moderate the pro-inflammatory action of excessive dietary omega-6consumption. If the amount of omega-3 fatty acids in the diet is increased, their metabolites (primarily EPA and DHA) compete with arachidonic acid for access to cyclooxygenase resulting increased production of anti-inflammatory mediators as well as a decrease in the pro-inflammatory mediators, thereby significantly reducing the ratio of pro-inflammatory to anti-inflammatory mediators.
In animal studies (rats) LA increases the size and number of tumors, whereas fish oil [containing the (n-3) fatty acids EPA and DHA] decreases the incidence of tumor formation, as well as tumor size.[131] This finding is consistent with other studies in rats that indicate that the potent inhibitors of prostaglandin synthesis, the NSAIDs indomethacin and flurbiprofen are effective at reducing the incidence of spontaneously occurring breast cancer. Epidemiological studies in humans have also indicated a potentially chemoprotective effect as result of long term consumption of NSAID drugs.[132-136] Fish oils have been used to adjust systemic levels of (n-3) fatty acids in animals models of colon, lung, breast, pancreatic and prostate cancers to reduce prostaglandin synthesis, with resulting chemoprevrention and/or slowed growth and metastases in neoplastic disease in the laboratory setting.[131]
These studies, together with the epidemiologic evidence, appear to confirm the importance of a (n-6) to (n-3) ratio of 2:1 as being chemoprotective in cancer, and raise the possibility that (n-3) fatty acids might be used as adjuvant therapy to reduce the risk of recurrence and metastases of breast cancer in humans following surgery and chemotherapy.[132-136] Epidemiological studies have also consistently shown that fish oil consumption protects against the development of a broad range of cancers, but especially breast and prostate cancer. [137-144] Thus, it is not the absolute level of either (n-3) or (n-6) lipids, but rather their presence in a ratio of 1:1 or 2:1 that chemoprotective against a number of cancers[128, 129, 145, 146] Western diets have a ratio of 10–20:1.[147]
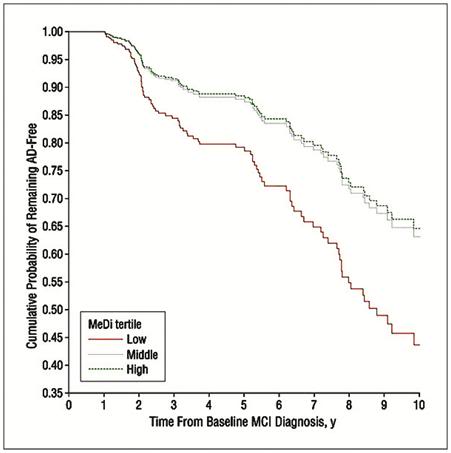
Figure 9: The Cretan diet provides significant protection against Alzheimer’s disease (AD) in patients who have been diagnosed with mild cognitive impairment (MCI). Survival curves based on Cox analysis comparing cumulative AD incidence in subjects with MCI at the first evaluation by Mediterranean diet (MeDi) adherence tertile (P for trend = .02). The figure is derived from a model that is adjusted for cohort, age, sex, ethnicity, education, APOE genotype, caloric intake, body mass index, and time between the first dietary assessment and the first cognitive assessment. Duration of follow-up is truncated at 10 years. Results of log-rank tests for pairwise comparisons are as follows: middle vs low tertile, 2 = 4.26, P = .03; low vs high tertile, 2 = 1.39, P = .23; and middle vs high tertile, 2 = 0.12, P = .72.[148, 149]
Both the Cretan and the Adventist vegetarian diets confer substantial protection against the mild cognitive impairment (MCI) of aging and against Alzheimer’s disease (AD)(Figure 9)[148, 149] Interestingly, the AHS-2 results demonstrate a link between the incidence of dementia and the consumption of all meat products, including fish and poultry. This may account for added benefit of Adventist Vegetarian diet over what would be expected on the basis of its lipid constituents and the presence of some adverse foodstuffs, such as refined sugar. Perhaps meat consumption is associated with adverse effects, per se? The literature is open to interpretation on this point.[150, 151]
The two highest quality studies examining the effect of vegetarian diets on lifespan, as well as morbidity were conducted by Key, et al., and were published in 2009.[152, 153] These studies found no significant difference in lifespan between the control and the vegetarian populations in the study. However, as so often happens in studies of this kind, both the control and the vegetarian group experienced statistically significant lower rates of mortality than the general population (UK). This kind of confounding result may be due to self-selection of on average healthier people within the general population to serve as controls. Another limitation on these studies is that they were barely powered adequately to detect small to moderate differences in mortality. The vegetarian group in this study had a lower body mass index (BMI) and consequently less obesity. The incidence of CVD and cancer were not statistically significant between the groups.
End of Part 2
References
1. Gottlieb SH: “K-ration”. Diabetes and cardiovascular risk. Diabetes Forecast 2002, 55(2):44-46.
2. Kalm LM, Semba RD: They starved so that others be better fed: remembering Ancel Keys and the Minnesota experiment. J Nutr 2005, 135(6):1347-1352.
3. Keys A, Taylor HL, Blackburn H, Brozek J, Anderson JT, Simonson E: Coronary Heart Disease among Minnesota Business and Professional Men Followed Fifteen Years. Circulation 1963, 28:381-395.
4. Skinner JD, Carruth BR, Pope J, Varner L, Goldberg D: Food and nutrient intake of white, pregnant adolescents. J Am Diet Assoc 1992, 92(9):1127-1129.
5. Grundmann E: Cancer morbidity and mortality in USA Mormons and Seventh-day Adventists. Arch Anat Cytol Pathol 1992, 40(2-3):73-78.
6. Fraser GE: Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am J Clin Nutr 1999, 70(3 Suppl):532S-538S.
7. Fraser GE: Determinants of ischemic heart disease in Seventh-day Adventists: a review. Am J Clin Nutr 1988, 48(3 Suppl):833-836.
8. Fraser GE, Lindsted KD, Beeson WL: Effect of risk factor values on lifetime risk of and age at first coronary event. The Adventist Health Study. Am J Epidemiol 1995, 142(7):746-758.
9. Fraser GE, Sabate J, Beeson WL, Strahan TM: A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med 1992, 152(7):1416-1424.
10. Fraser GE, Shavlik DJ: Ten years of life: Is it a matter of choice? Arch Intern Med 2001, 161(13):1645-1652.
11. Fraser GE, Shavlik DJ: Risk factors for all-cause and coronary heart disease mortality in the oldest-old. The Adventist Health Study. Arch Intern Med 1997, 157(19):2249-2258.
12. Giem P, Beeson WL, Fraser GE: The incidence of dementia and intake of animal products: preliminary findings from the Adventist Health Study. Neuroepidemiology 1993, 12(1):28-36.
13. Mills PK, Beeson WL, Phillips RL, Fraser GE: Cancer incidence among California Seventh-Day Adventists, 1976-1982. Am J Clin Nutr 1994, 59(5 Suppl):1136S-1142S.
14. Singh PN, Sabate J, Fraser GE: Does low meat consumption increase life expectancy in humans? Am J Clin Nutr 2003, 78(3 Suppl):526S-532S.
15. Snowdon DA, Phillips RL, Fraser GE: Meat consumption and fatal ischemic heart disease. Prev Med 1984, 13(5):490-500.
16. Mills PK, Preston-Martin S, Annegers JF, Beeson WL, Phillips RL, Fraser GE: Risk factors for tumors of the brain and cranial meninges in Seventh-Day Adventists. Neuroepidemiology 1989, 8(5):266-275.
17. Singh N, Graves J, Taylor PD, MacAllister RJ, Singer DR: Effects of a ‘healthy’ diet and of acute and long-term vitamin C on vascular function in healthy older subjects. Cardiovasc Res 2002, 56(1):118-125.
18. Taylor CB, Allen ES, Mikkelson B, Kang-Jey H: Serum cholesterol levels of Seventh-day Adventists. Paroi Arterielle 1976, 3(4):175-179.
19. Kahn HA, Phillips RL, Snowdon DA, Choi W: Association between reported diet and all-cause mortality. Twenty-one-year follow-up on 27,530 adult Seventh-Day Adventists. Am J Epidemiol 1984, 119(5):775-787.
20. Beeson WL, Mills PK, Phillips RL, Andress M, Fraser GE: Chronic disease among Seventh-day Adventists, a low-risk group. Rationale, methodology, and description of the population. Cancer 1989, 64(3):570-581.
21. Masley SC, Weaver W, Peri G, Phillips SE: Efficacy of lifestyle changes in modifying practical markers of wellness and aging. Altern Ther Health Med 2008, 14(2):24-29.
22. Mills PK, Annegers JF, Phillips RL: Animal product consumption and subsequent fatal breast cancer risk among Seventh-day Adventists. Am J Epidemiol 1988, 127(3):440-453.
23. Phillips RL, Garfinkel L, Kuzma JW, Beeson WL, Lotz T, Brin B: Mortality among California Seventh-Day Adventists for selected cancer sites. J Natl Cancer Inst 1980, 65(5):1097-1107.
24. Phillips RL, Snowdon DA: Dietary relationships with fatal colorectal cancer among Seventh-Day Adventists. J Natl Cancer Inst 1985, 74(2):307-317.
25. Vang A, Singh PN, Lee JW, Haddad EH, Brinegar CH: Meats, processed meats, obesity, weight gain and occurrence of diabetes among adults: findings from Adventist Health Studies. Ann Nutr Metab 2008, 52(2):96-104.
26. Fonnebo V: The Tromso Heart Study: coronary risk factors in Seventh-Day Adventists. Am J Epidemiol 1985, 122(5):789-793.
27. Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S et al: Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med 1995, 155(4):381-386.
28. Coronary heart disease in seven countries. I. The study program and objectives. Circulation 1970, 41(4 Suppl):I1-8.
29. Twenty-five year incidence and prediction of coronary heart disease in two Italian rural population samples. The Italian Research Group of the Seven Countries Study. Acta Cardiol 1986, 41(4):283-299.
30. The diet and all-causes death rate in the Seven Countries Study. Lancet 1981, 2(8237):58-61.
31. Alberti-Fidanza A, Fidanza F, Chiuchiu MP, Verducci G, Fruttini D: Dietary studies on two rural italian population groups of the Seven Countries Study. 3. Trend Of food and nutrient intake from 1960 to 1991. Eur J Clin Nutr 1999, 53(11):854-860.
32. Alberti-Fidanza A, Paolacci CA, Chiuchiu MP, Coli R, Fruttini D, Verducci G, Fidanza F: Dietary studies on two rural Italian population groups of the Seven Countries Study. 1. Food and nutrient intake at the thirty-first year follow-up in 1991. Eur J Clin Nutr 1994, 48(2):85-91.
33. Alberti-Fidanza A, Paolacci CA, Chiuchiu MP, Coli R, Parretta MG, Verducci G, Fidanza F: Dietary studies on two rural Italian population groups of the Seven Countries Study. 2. Concurrent validation of protein, fat and carbohydrate intake. Eur J Clin Nutr 1994, 48(2):92-96.
34. Alonso A, Jacobs DR, Jr., Menotti A, Nissinen A, Dontas A, Kafatos A, Kromhout D: Cardiovascular risk factors and dementia mortality: 40 years of follow-up in the Seven Countries Study. J Neurol Sci 2009, 280(1-2):79-83.
35. Alvarez Guisasola F, Mavros P, Nocea G, Alemao E, Alexander CM, Yin D: Glycaemic control among patients with type 2 diabetes mellitus in seven European countries: findings from the Real-Life Effectiveness and Care Patterns of Diabetes Management (RECAP-DM) study. Diabetes Obes Metab 2008, 10 Suppl 1:8-15.
36. Cheng TO: Ancel Keys, seven countries study, China connections, and K rations. J Am Diet Assoc 2005, 105(3):349.
37. de Lorgeril M, Salen P: Mediterranean type of diet for the prevention of coronary heart disease. A global perspective from the seven countries study to the most recent dietary trials. Int J Vitam Nutr Res 2001, 71(3):166-172.
38. Ducimetiere P, Richard JL, Cambien F, Rakotovao R, Claude JR: Coronary heart disease in middle-aged Frenchmen. Comparisons between Paris Prospective Study, Seven Countries Study, and Pooling Project. Lancet 1980, 1(8182):1346-1350.
39. Farchi G, Fidanza F, Giampaoli S, Mariotti S, Menotti A: Alcohol and survival in the Italian rural cohorts of the Seven Countries Study. Int J Epidemiol 2000, 29(4):667-671.
40. Feskens EJ, Tuomilehto J, Stengard JH, Pekkanen J, Nissinen A, Kromhout D: Hypertension and overweight associated with hyperinsulinaemia and glucose tolerance: a longitudinal study of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetologia 1995, 38(7):839-847.
41. Feskens EJ, Virtanen SM, Rasanen L, Tuomilehto J, Stengard J, Pekkanen J, Nissinen A, Kromhout D: Dietary factors determining diabetes and impaired glucose tolerance. A 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetes Care 1995, 18(8):1104-1112.
42. Fidanza F, Alberti A, Lanti M, Menotti A: Mediterranean diet score: correlation with 25-year mortality from coronary heart disease in the Seven Countries Study. Nutr Metab Cardiovasc Dis 2004, 14(6):397.
43. Alberti A, Fruttini D, Fidanza F: The Mediterranean Adequacy Index: further confirming results of validity. Nutr Metab Cardiovasc Dis 2009, 19(1):61-66.
44. Jacobs DR, Jr., Adachi H, Mulder I, Kromhout D, Menotti A, Nissinen A, Blackburn H: Cigarette smoking and mortality risk: twenty-five-year follow-up of the Seven Countries Study. Arch Intern Med 1999, 159(7):733-740.
45. Jansen DF, Nedeljkovic S, Feskens EJ, Ostojic MC, Grujic MZ, Bloemberg BP, Kromhout D: Coffee consumption, alcohol use, and cigarette smoking as determinants of serum total and HDL cholesterol in two Serbian cohorts of the Seven Countries Study. Arterioscler Thromb Vasc Biol 1995, 15(11):1793-1797.
46. Jansen MC, Bueno-de-Mesquita HB, Buzina R, Fidanza F, Menotti A, Blackburn H, Nissinen AM, Kok FJ, Kromhout D: Dietary fiber and plant foods in relation to colorectal cancer mortality: the Seven Countries Study. Int J Cancer 1999, 81(2):174-179.
47. Jansen MC, Bueno-de-Mesquita HB, Rasanen L, Fidanza F, Menotti A, Nissinen A, Feskens EJ, Kok FJ, Kromhout D: Consumption of plant foods and stomach cancer mortality in the seven countries study. Is grain consumption a risk factor? Seven Countries Study Research Group. Nutr Cancer 1999, 34(1):49-55.
48. Kafatos A, Diacatou A, Voukiklaris G, Nikolakakis N, Vlachonikolis J, Kounali D, Mamalakis G, Dontas AS: Heart disease risk-factor status and dietary changes in the Cretan population over the past 30 y: the Seven Countries Study. Am J Clin Nutr 1997, 65(6):1882-1886.
49. Karon JM, Parker RA: Re: “The diet and 15-year death rate in the Seven Countries Study”. Am J Epidemiol 1988, 128(1):238-241.
50. Keys A, Menotti A, Aravanis C, Blackburn H, Djordevic BS, Buzina R, Dontas AS, Fidanza F, Karvonen MJ, Kimura N et al: The seven countries study: 2,289 deaths in 15 years. Prev Med 1984, 13(2):141-154.
51. Kromhout D: [The 'Seven Countries Study': 40 years of research in coronary heart diseases in 7 countries]. Ned Tijdschr Geneeskd 1997, 141(1):7-9.
52. Kromhout D, Bloemberg B, Feskens E, Menotti A, Nissinen A: Saturated fat, vitamin C and smoking predict long-term population all-cause mortality rates in the Seven Countries Study. Int J Epidemiol 2000, 29(2):260-265.
53. Kromhout D, Bloemberg B, Seidell JC, Nissinen A, Menotti A: Physical activity and dietary fiber determine population body fat levels: the Seven Countries Study. Int J Obes Relat Metab Disord 2001, 25(3):301-306.
54. Kromhout D, Katan MB, Menotti A, Keys A, Bloemberg B: Serum cholesterol and long-term death rates from suicide, accidents, or violence. Seven Countries Study Group. Lancet 1992, 340(8814):317.
55. Kromhout D, Menotti A, Bloemberg B, Aravanis C, Blackburn H, Buzina R, Dontas AS, Fidanza F, Giampaoli S, Jansen A et al: Dietary saturated and trans fatty acids and cholesterol and 25-year mortality from coronary heart disease: the Seven Countries Study. Prev Med 1995, 24(3):308-315.
56. Kromhout D, Nedeljkovic SI, Grujic MZ, Ostojic MC, Keys A, Menotti A, Katan MB, van Oostrom MA, Bloemberg BP: Changes in major risk factors for cardiovascular diseases over 25 years in the Serbian cohorts of the Seven Countries Study. Int J Epidemiol 1994, 23(1):5-11.
57. Lanti M, Menotti A, Nedeljkovic S, Nissinen A, Kafatos A, Kromhout D: Long-term trends in major cardiovascular risk factors in cohorts of aging men in the European cohorts of the Seven Countries Study. Aging Clin Exp Res 2005, 17(4):306-315.
58. Mamalakis G, Jansen E, Cremers H, Kiriakakis M, Tsibinos G, Kafatos A: Depression and adipose and serum cholesteryl ester polyunsaturated fatty acids in the survivors of the seven countries study population of Crete. Eur J Clin Nutr 2006, 60(8):1016-1023.
59. Mamalakis G, Kiriakakis M, Tsibinos G, Kafatos A: Depression and adipose polyunsaturated fatty acids in the survivors of the Seven Countries Study population of Crete. Prostaglandins Leukot Essent Fatty Acids 2004, 70(6):495-501.
60. Mariotti S, Capocaccia R, Farchi G, Menotti A, Verdecchia A, Keys A: Differences in the incidence rate of coronary heart disease between north and south European cohorts of the Seven Countries Study as partially explained by risk factors. Eur Heart J 1982, 3(5):481-487.
61. Mariotti S, Capocaccia R, Farchi G, Menotti A, Verdecchia A, Keys A: Age, period, cohort and geographical area effects on the relationship between risk factors and coronary heart disease mortality. 15-year follow-up of the European cohorts of the Seven Countries study. J Chronic Dis 1986, 39(3):229-242.
62. Menotti A: The relationship of total serum cholesterol to coronary heart disease in older men. The Italian rural areas of the Seven Countries Study. Ann Epidemiol 1992, 2(1-2):107-111.
63. Menotti A, Blackburn H, Kromhout D, Nissinen A, Adachi H, Lanti M: Cardiovascular risk factors as determinants of 25-year all-cause mortality in the seven countries study. Eur J Epidemiol 2001, 17(4):337-346.
64. Menotti A, Blackburn H, Kromhout D, Nissinen A, Karvonen M, Aravanis C, Dontas A, Fidanza F, Giampaoli S: The inverse relation of average population blood pressure and stroke mortality rates in the seven countries study: a paradox. Eur J Epidemiol 1997, 13(4):379-386.
65. Menotti A, Blackburn H, Seccareccia F, Kromhout D, Nissinen A, Aravanis C, Giampaoli S, Mohacek I, Nedeljkovic S, Toshima H: The relation of chronic diseases to all-cause mortality risk–the Seven Countries Study. Ann Med 1997, 29(2):135-141.
66. Menotti A, Conti S, Corradini P, Giampaoli S, Rumi A, Signoretti P: [Incidence and prediction of coronary heart disease in the Italian cohorts of the Seven Countries Study. 10 year experience (author's transl)]. G Ital Cardiol 1980, 10(7):792-806.
67. Menotti A, Jacobs DR, Jr., Blackburn H, Kromhout D, Nissinen A, Nedeljkovic S, Buzina R, Mohacek I, Seccareccia F, Giampaoli S et al: Twenty-five-year prediction of stroke deaths in the seven countries study: the role of blood pressure and its changes. Stroke 1996, 27(3):381-387.
68. Menotti A, Keys A, Aravanis C, Blackburn H, Dontas A, Fidanza F, Karvonen MJ, Kromhout D, Nedeljkovic S, Nissinen A et al: Seven Countries Study. First 20-year mortality data in 12 cohorts of six countries. Ann Med 1989, 21(3):175-179.
69. Menotti A, Keys A, Blackburn H, Aravanis C, Dontas A, Fidanza F, Giampaoli S, Karvonen M, Kromhout D, Nedeljkovic S et al: Twenty-year stroke mortality and prediction in twelve cohorts of the Seven Countries Study. Int J Epidemiol 1990, 19(2):309-315.
70. Menotti A, Keys A, Blackburn H, Karvonen M, Punsar S, Nissinen A, Pekkanen J, Kromhout D, Giampaoli S, Seccareccia F et al: Blood pressure changes as predictors of future mortality in the seven countries study. J Hum Hypertens 1991, 5(3):137-144.
71. Menotti A, Keys A, Blackburn H, Kromhout D, Karvonen M, Nissinen A, Pekkanen J, Punsar S, Fidanza F, Giampaoli S et al: Comparison of multivariate predictive power of major risk factors for coronary heart diseases in different countries: results from eight nations of the Seven Countries Study, 25-year follow-up. J Cardiovasc Risk 1996, 3(1):69-75.
72. Menotti A, Keys A, Kromhout D, Blackburn H, Aravanis C, Bloemberg B, Buzina R, Dontas A, Fidanza F, Giampaoli S et al: Inter-cohort differences in coronary heart disease mortality in the 25-year follow-up of the seven countries study. Eur J Epidemiol 1993, 9(5):527-536.
73. Allbaugh L: Crete: A Case Study of an Underdeveloped Area. Princeton, N.J: Princeton University Press; 1953.
74. Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys MH et al: The diet and 15-year death rate in the seven countries study. Am J Epidemiol 1986, 124(6):903-915.
75. Simopoulos A, Robinson, J.: The Omega Diet. The Lifesaving Nutritional Program Based on the Diet of the Island of Crete. New York: HarperCollins; 1999.
76. Simopoulos AP: The traditional diet of Greece and cancer. Eur J Cancer Prev 2004, 13(3):219-230.
77. Simopoulos AP: What is so special about the diet of Greece? The scientific evidence. World Rev Nutr Diet 2005, 95:80-92.
78. Ornish D: Can lifestyle changes reverse coronary heart disease? World Rev Nutr Diet 1993, 72:38-48.
79. Hubbard J, Inkeles, S, Barnard, RJ.: Nathan Pritikin’s Heart. N Engl J Med 1985, 313:52.
80. Barnard R, Pritikin, R, Rosenthal, R, et al.: Pritikin Approach to Cardiac Rehabilitation; Rehabilitation Medicine. St. Louis: Mosby Company, ; 1988.
81. Katan M, Beynen, AC.: Linoleic acid consumption and coronary heart disease in U.S.A. and U.K. Lancet 1981, 15(2(8242)):371.
82. Goldman L, Cook, EF.: The decline in ischemic heart disease mortality rates: An analysis of the comparative effects of medical interventions and changes in lifestyle. . Ann Intern Med 1984 101(6)::825-836.
83. Keys A: Coronary heart disease in seven countries. Circulation
1970, 41: (suppl.): 1-211
84. Keyes A: Coronary heart disease in seven countries. Circulation
1970, 41: (suppl.): 1-211
85. Sandker GW, Kromhout, D, Aravanis, C, Bloemberg, BP. Mensink, R P, Karalias, N, Katan, MB.: Serum lipids in elderly men in Crete and The Netherlands. . Eur J Clin Nutr 1993, 47:201-208.
86. Willett W: Lessons from dietary studies in Adventists and questions for the future. Am J Clin Nutr 2003, 78(3 Suppl):539S-543S.
87. de Lorgeril M, Salen P: The Mediterranean diet in secondary prevention of coronary heart disease. Clin Invest Med 2006, 29(3):154-158.
88. Rudel L, Parks, JS, Sawyer, JK.: Compared with dietary monounsaturated and saturated fat, polyunsaturated fat protects african green monkeys from coronary artery atherosclerosis Arteriosclerosis, Thrombosis, and Vascular Biology 1995, 15:2101-2110
89. Higashi K, Ishikawa, T, Shige H, Tomiyasu, K, Yoshida, H, Ito, T, Nakajima, K, Yonemura, A, Sawada, S, Nakamura, H.: Olive oil increases the magnitude of postprandial chylomicron remnants compared to milk fat and safflower oil. J Am Coll Nutr 1997, 16::429-434.
90. de Bruin T, Brouwer, CB, van Linde-Sibenius, TM, Erkelens,DW. : Different postprandial metabolism of olive oil and soybean oil: a possible mechanism of the high-density lipoprotein conserving effect of olive oil. Am J Clin Nutr 1993, 58:477-483.
91. Bray G, Popkin, BM. : Dietary fat intake does affect obesity. Am J Clin Nutr 1998, 68:1157-1173.
92. Briante R, Febbraio F, Nucci R: Antioxidant properties of low molecular weight phenols present in the mediterranean diet. J Agric Food Chem 2003, 51(24):6975-6981.
93. Boskou D: Olive oil. World Rev Nutr Diet 2007, 97:180-210.
94. Konstantinidou V, Covas MI, Munoz-Aguayo D, Khymenets O, de la Torre R, Saez G, Tormos Mdel C, Toledo E, Marti A, Ruiz-Gutierrez V et al: In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: a randomized controlled trial. FASEB J, 24(7):2546-2557.
95. Wahle KW, Caruso D, Ochoa JJ, Quiles JL: Olive oil and modulation of cell signaling in disease prevention. Lipids 2004, 39(12):1223-1231.
96. Battino M, Ferreiro MS: Ageing and the Mediterranean diet: a review of the role of dietary fats. Public Health Nutr 2004, 7(7):953-958.
97. Manios Y, Detopoulou V, Visioli F, Galli C: Mediterranean diet as a nutrition education and dietary guide: misconceptions and the neglected role of locally consumed foods and wild green plants. Forum Nutr 2006, 59:154-170.
98. Ferro-Luzzi A, James WP, Kafatos A: The high-fat Greek diet: a recipe for all? Eur J Clin Nutr 2002, 56(9):796-809.
99. Renaud S, de Lorgeril, M., Delaye, J., Guidollet, J., Jacquard, F., Mamelle, N., Martin, J. L., Monjaud, I., Salen, P, Touboul, P.: Cretan
Mediterranean diet for prevention of coronary heart disease. Am J Clin Nutr 1995, 61((suppl.)):1360S-1367S.
100. Mamelle N: Mediterranean dietary pattern in a randomized trial. Prolonged survival and possible reduced cancer rate. Arch Intern Med 1998, 158:1181-1187.
101. de Lorgeril M, Salen P: Modified cretan Mediterranean diet in the prevention of coronary heart disease and cancer: An update. World Rev Nutr Diet 2007, 97:1-32.
102. Simopoulos A: Omega-3 fatty acids in health and disease and in growth and development. m J Clin Nutr 1991(54):438-463.
103. Freese R, Mutanen M, Valsta LM, Salminen I: Comparison of the effects of two diets rich in monounsaturated fatty acids differing in their linoleic/alpha-linolenic acid ratio on platelet aggregation. Thromb Haemost 1994, 71(1):73-77.
104. Valsta LM, Salminen I, Aro A, Mutanen M: Alpha-linolenic acid in rapeseed oil partly compensates for the effect of fish restriction on plasma long chain n-3 fatty acids. Eur J Clin Nutr 1996, 50(4):229-235.
105. Sebedio JL, Vermunt SH, Chardigny JM, Beaufrere B, Mensink RP, Armstrong RA, Christie WW, Niemela J, Henon G, Riemersma RA: The effect of dietary trans alpha-linolenic acid on plasma lipids and platelet fatty acid composition: the TransLinE study. Eur J Clin Nutr 2000, 54(2):104-113.
106. Garcia-Marcos L, Canflanca IM, Garrido JB, Varela AL, Garcia-Hernandez G, Guillen Grima F, Gonzalez-Diaz C, Carvajal-Uruena I, Arnedo-Pena A, Busquets-Monge RM et al: Relationship of asthma and rhinoconjunctivitis with obesity, exercise and Mediterranean diet in Spanish schoolchildren. Thorax 2007, 62(6):503-508.
107. Burri BJ, Dougherty RM, Kelley DS, Iacono JM: Platelet aggregation in humans is affected by replacement of dietary linoleic acid with oleic acid. Am J Clin Nutr 1991, 54(2):359-362.
108. Chan JK, McDonald BE, Gerrard JM, Bruce VM, Weaver BJ, Holub BJ: Effect of dietary alpha-linolenic acid and its ratio to linoleic acid on platelet and plasma fatty acids and thrombogenesis. Lipids 1993, 28(9):811-817.
109. Birch E, Hoffman, DR, Uauy, R, Birch, DG, Prestidge, C.: Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatr Res 1998, 44: 201-209.
110. Birch E, Birch, D., Hoffman, D., Hale, L., Everett, M, Uauy, R.: Breast-feeding and optimal visual development Strabismus
J Pediatr Ophthalmol 1993, 30: 33-38.
111. Simopoulos A, Kifer, RR, Martin, RE.: Health effects of polyunsaturated fatty acids in seafoods. Orlando: Academic Press; 1986.
112. Hamazaki T, Okuyama, H. (ed.): Fatty Acids and Lipids-New Findings: http://content.karger.com/ProdukteDB/produkte.asp?Aktion=showproducts&searchWhat=books&searchParm=toc&ProduktNr=224409. Basel: Karger; 2001.
113. Leaf A, Weber, PC.: Cardiovascular effects of n-3 fatty acids. N Engl J Med 1988, 318:549-557.
114. Simopoulos A: Fatty acids in the prevention-management of cardiovascular disease. Can J Physiol Pharmacol 1997, 75:234-239.
115. Simopoulos A: Essential fatty acids in health and chronic disease. Am J Clin Nutr 1999, 70 ( (suppl.)):560S-569S.
116. Simopoulos A, Leaf, A, Salem, N., Jr.: Essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Ann Nutr Metabolism 1999, 43:27-130.
117. Weisburger J: Mechanisms of action in antioxidants as exemplified in vegetables, tomatoes and tea. Food Chem Toxicol 1999, 37(943-948).
118. Hertog M, Hollman, PCH, Katan, MB.: Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in The Netherlands. J Agric Food Chem 1992, 40:2379-2383.
119. Slater T, Block, G, eds.: Antioxidant vitamins and b-carotene in disease prevention. Am J Clin Nutr 1991, 53( (suppl.)):189S-396S.
120. Agradi E, Vegeto E, Sozzi A, Fico G, Regondi S, Tome F: Traditional healthy Mediterranean diet: estrogenic activity of plants used as food and flavoring agents. Phytother Res 2006, 20(8):670-675.
121. Gerber M: Biofactors in the Mediterranean diet. Clin Chem Lab Med 2003, 41(8):999-1004.
122. Heinrich M, Leonti M, Nebel S, Peschel W: “Local Food – Nutraceuticals”: an example of a multidisciplinary research project on local knowledge. J Physiol Pharmacol 2005, 56 Suppl 1:5-22.
123. Visioli F, Galli C: Biological properties of olive oil phytochemicals. Crit Rev Food Sci Nutr 2002, 42(3):209-221.
124. Cristina F: Mediterranean diet health benefits may be due to a synergistic combination of phytochemicals and fatty-acids. BMJ 2005, 331(7508):E366.
125. Mills PK, Beeson WL, Phillips RL, Fraser GE: Bladder cancer in a low risk population: results from the Adventist Health Study. Am J Epidemiol 1991, 133(3):230-239.
126. Hirayama T: Mortality in Japanese with life-styles similar to Seventh-Day Adventists: strategy for risk reduction by life-style modification. Natl Cancer Inst Monogr 1985, 69:143-153.
127. Zollinger TW, Phillips RL, Kuzma JW: Breast cancer survival rates among Seventh-day Adventists and non-Seventh-day Adventists. Am J Epidemiol 1984, 119(4):503-509.
128. Levi F, La Vecchia, C, Lucchini, F, Negri, E.: Cancer mortalityin Europe. Eur J Cancer Prev 1995, 1990-1992(4):389-417.
129. Stamatiou K, Alevizos A, Mariolis A, Sofras F: [Mediterranean diet and prostate cancer]. Actas Urol Esp 2006, 30(3):340.
130. Fernandez E, Gallus S, La Vecchia C: Nutrition and cancer risk: an overview. J Br Menopause Soc 2006, 12(4):139-142.
131. Cave W, Jr.: Fatty acid diet effects on tumorigenesis in experimental animals. . World Rev Nutr Diet 1991, 3(66):462-476.
132. Cowing B, Saker, KE.: Polyunsaturated fatty acids and epidermal growth factor receptor/mitogen-activated protein kinase signaling in
mammary cancer. J Nutr 2001, 131::1125-1128.
133. Schley PD, Brindley DN, Field CJ: (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J Nutr 2007, 137(3):548-553.
134. Ge Y, Chen Z, Kang ZB, Cluette-Brown J, Laposata M, Kang JX: Effects of adenoviral gene transfer of C. elegans n-3 fatty acid desaturase on the lipid profile and growth of human breast cancer cells. Anticancer Res 2002, 22(2A):537-543.
135. Bardon S, Le MT, Alessandri JM: Metabolic conversion and growth effects of n-6 and n-3 polyunsaturated fatty acids in the T47D breast cancer cell line. Cancer Lett 1996, 99(1):51-58.
136. Chajes V, Sattler W, Stranzl A, Kostner GM: Influence of n-3 fatty acids on the growth of human breast cancer cells in vitro: relationship to peroxides and vitamin-E. Breast Cancer Res Treat 1995, 34(3):199-212.
137. Nielsen N, Hansen, JPH.: Breast cancer in Greenland-selected epidemiological, clinical and histological features. J Cancer Res Clin Oncol 1980, 98:287-299.
138. Terry P, Lichtenstein, P, Feychting, M, Ahlbom, A, Wolk, A.: Fatty fish consumption and risk of prostate cancer. Lancet 2001, 357:1764-1766.
139. Wargovich MJ: Fish oil and colon cancer. Gastroenterology 1992, 103(3):1096-1098.
140. Caygill CP, Charlett A, Hill MJ: Fat, fish, fish oil and cancer. Br J Cancer 1996, 74(1):159-164.
141. Lindner MA: A fish oil diet inhibits colon cancer in mice. Nutr Cancer 1991, 15(1):1-11.
142. Mandal CC, Ghosh-Choudhury T, Yoneda T, Choudhury GG, Ghosh-Choudhury N: Fish oil prevents breast cancer cell metastasis to bone. Biochem Biophys Res Commun, 402(4):602-607.
143. Moreira AP, Sabarense CM, Dias CM, Lunz W, Natali AJ, Gloria MB, Peluzio MC: Fish oil ingestion reduces the number of aberrant crypt foci and adenoma in 1,2-dimethylhydrazine-induced colon cancer in rats. Braz J Med Biol Res 2009, 42(12):1167-1172.
144. Chiang KC, Persons KS, Istfan NW, Holick MF, Chen TC: Fish oil enhances the antiproliferative effect of 1alpha,25-dihydroxyvitamin D3 on liver cancer cells. Anticancer Res 2009, 29(9):3591-3596.
145. Miano L: [Mediterranean diet, micronutrients and prostate carcinoma: a rationale approach to primary prevention of prostate cancer]. Arch Ital Urol Androl 2003, 75(3):166-178.
146. Yee LD, Young DC, Rosol TJ, Vanbuskirk AM, Clinton SK: Dietary (n-3) polyunsaturated fatty acids inhibit HER-2/neu-induced breast cancer in mice independently of the PPARgamma ligand rosiglitazone. J Nutr 2005, 135(5):983-988.
147. Simopoulos A: Evolutionary aspects of diet and essential fatty acids. World Rev Nutr Diet 2001, 88:18-27.
148. Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA: Mediterranean diet and mild cognitive impairment. Arch Neurol 2009, 66(2):216-225.
149. Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA: Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol 2006, 59(6):912-921.
150. Appleby PN, Thorogood M, Mann JI, Key TJ: The Oxford Vegetarian Study: an overview. Am J Clin Nutr 1999, 70(3 Suppl):525S-531S.
151. Key TJ, Appleby PN, Rosell MS: Health effects of vegetarian and vegan diets. Proc Nutr Soc 2006, 65(1):35-41.
152. Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE: Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am J Clin Nutr 2009, 89(5):1613S-1619S.
153. Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE: Cancer incidence in vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am J Clin Nutr 2009, 89(5):1620S-1626S.

Sadly, this information is a bit outdated. Ancel Keys data has since been expanded to cover 24 countries proving his assertions off base. Recently significant problems have been found with studies used by the American Heart Association in creating the advisory to decrease saturated fat and increase polyunsaturated fat (without regard for the proper balance between omega-6 and omega-3 fatty acids in the blood and tissues) and irresponsibly suggesting a high level of omega-6 intake (not supported by the research). I highly suggest the latest: http://www.bmj.com/content/346/bmj.e8707.
Happy to see an increasing awareness of the need to balance omega-6 and omega-3. Thank you for that! I believe one of the many great benefits of olive oil must be the relatively small amount of omega-6 it contains, or at least the low ratio of omega-6 to omega-3.