By Mike Darwin
A Great Team
In January of 1980 I stabilized and transported a patient from southern Wisconsin for TT to Cryovita Labs. Jerry kindly invited me to stay and participate in this patient’s cryoprotective perfusion (CPA) perfusion and cool down and I readily accepted.
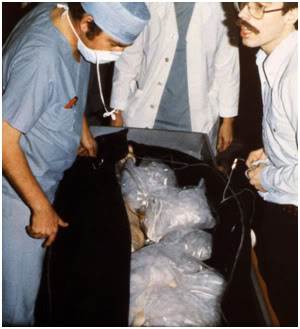 Figure 15: Jerry Leaf at left and Mike Darwin at right performing the initial examination of a patient stabilized remotely in Wisconsin and Transported to the facilities of Cryovita Laboratories in 1980.
Figure 15: Jerry Leaf at left and Mike Darwin at right performing the initial examination of a patient stabilized remotely in Wisconsin and Transported to the facilities of Cryovita Laboratories in 1980.
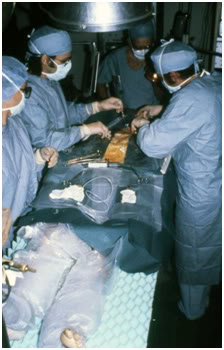 Figure 16: Patient undergoing median sternotomy prior to cannulation for cryoprotective perfusion at Cryovita Laboratories in 1980.
Figure 16: Patient undergoing median sternotomy prior to cannulation for cryoprotective perfusion at Cryovita Laboratories in 1980.
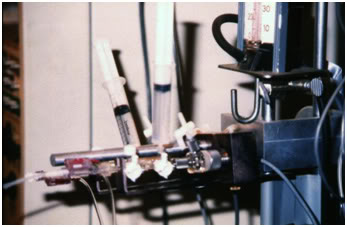 Figure 17: Intravascular pressure monitoring set-up (Intraflow™, stopcock manifold, Statham pressure transducer and calibrating mercury manometer) as used to monitor cryopatient arterial and central venous pressure at Cryovita Laboratories in 1980.
Figure 17: Intravascular pressure monitoring set-up (Intraflow™, stopcock manifold, Statham pressure transducer and calibrating mercury manometer) as used to monitor cryopatient arterial and central venous pressure at Cryovita Laboratories in 1980.
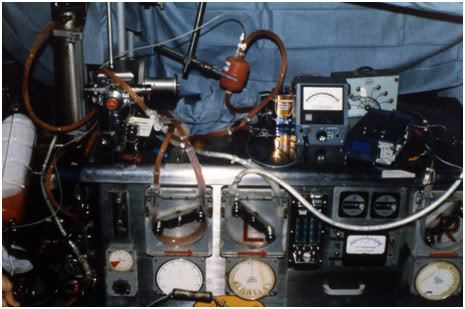 Figure 18: Heart-lung machine and extracorporeal circuit employed to carry out cryoprotective perfusion of cryopatients at Cryovita Laboratories in 1980.
Figure 18: Heart-lung machine and extracorporeal circuit employed to carry out cryoprotective perfusion of cryopatients at Cryovita Laboratories in 1980.
As we were finishing up this case, another patient, a long-time member of the Bay Area Cryonics Society, experienced unexpected cardiac arrest and presented for cryopreservation. With almost no sleep, and virtually no turnaround time between cases, this patient was cryopreserved, as well. A detailed technical report on both of these cases was published in Cryonics magazine in 1985.12
Doing those two cases with Jerry convinced me that that the most effective and important action I could take to both further cryonics (and improve my own skills) was to shutter the now considerable cryonics operations in Indianapolis, and relocate to work with Jerry in Southern California. That is exactly what I did in the spring of 1981. My reasons for this are best articulated by Jerry in an interview he gave in August of 1986:
“In 1980 I had the occasion to make personal contact with Mike Federowicz, who I had corresponded with before. Mike had transported a Trans Time patient to Southern California and then stayed on to help with a second suspension which came on the heels of the first. Mike had been working in a cryonics group in Indianapolis, Indiana for a number of years. At that time I tried to open the door as far as doing what I could to persuade him that Southern California offered an attractive alternative to the difficulties he was experiencing in Indiana. I needed someone else out here to work with who had a background in clinical medicine, such as Mike did, and he himself had begun to move toward clinical models of perfusion — using roller pumps and so on. I felt that he and I working together would allow us both to accomplish a lot more than if we were working alone. He was the only one else in the world who seemed to be aware of the fact that something needed to be done to upgrade the level of care — and to realize that that meant medical technology.”13
Throughout the early and mid-1980s – often under very trying conditions, and with virtually no money other than that which we earned ourselves, Jerry, I and the small band of committed cryonicists that comprised Alcor at that time, relentlessly accumulated equipment and conducted basic research to determine just how effective (or ineffective) then current cryopreservation techniques were at preserving both viability and structure. We also began the very successful canine total body washout (TBW or blood washout) and extended ultraprofound hypothermic asanguineous perfusion research work which culminated in consistent recovery of the animals from 4 hours of perfusion at ~5oC.14 Our thanks for that was not infrequent harsh criticism, most of which emanated from our fellow cryonicists.15,16
 Figure 19: Shiley pediatric blood oxygenator (bubbler) and blood reservoir bags used to hold the animal’s blood during asanguineous perfusion at Cryovita Laboratories on 17 March, 1984. The stainless steel basin was used to collect discard effluent at the tail end of blood washout and replacement.
Figure 19: Shiley pediatric blood oxygenator (bubbler) and blood reservoir bags used to hold the animal’s blood during asanguineous perfusion at Cryovita Laboratories on 17 March, 1984. The stainless steel basin was used to collect discard effluent at the tail end of blood washout and replacement.
Over the past two years, this work has been has been decried as useless, scientifically invalid, and it has been implied it was conducted in an unlicensed facility, using illegally obtained animals. In fact, Cryovita was a USDA[1] licensed facility from well before the time that any animal (other than the human kind) entered the premises. Jerry was inordinately proud of his USDA license, and Cryovita was inspected frequently by the USDA, and never had a serious violation. At the time this research began in the late 1970s and early 1980s, ‘pound seizure’ was an operational and fully legal mechanism for acquiring dogs and cats for research.
While still technically legal in many states, including California,17 pound seizure has effectively been all but abolished, and the result is that each year, according to the US Humane Society, 6-8 million dogs and cats are killed; to no purpose but to dispose of them. I have personally witnessed this process at a variety of facilities and most of the animals die in terror and confusion – many having been injured by others of their species while ‘timing-out’ in local government custody. Their deaths serve no purpose but to dispose of a suffering and ‘unwanted’ commodity produced by irresponsible and cruel people. The majority of animal research facilities at the time when pound seizure was operational provided exemplary and kind care for the animals they used for experimental purposes. In fact, such care is essential to good research, because a stressed, malnourished and unconditioned animal will yield suspect or meaningless experimental results.
Thus, experimental animals obtained from the pound, or from licensed dealers or breeders must be wormed, vaccinated, well fed and emotionally supported prior to use in experimental procedures. Just as much to the point, the vast majority (>90%) of such procedures employing dogs and cats are ‘acute procedures.’ That means that the animal is placed in deep anesthesia and never awakens from the procedure. In practice, this means additional weeks of life for the animal under humane conditions, and a death that is virtually identical to that it would have experienced if ‘euthanized’ in the pound.
Chronic studies are carefully regulated, and an animal can be used only once for any such study, and indeed, for any experimental procedure.18 A consequence of the Animal Rights Activists’ intervention to prevent pound seizure has been the useless and irresponsible deaths of millions of discarded animals. And keep in mind that one of the biggest beneficiaries of animal research are arguably the animals themselves – since many procedures targeted for human use cannot jump the FDA regulatory or marketing hurdle for human application – but can do so for animals. Joint replacement, NF-kappa B non-steroidal chronic pain drugs such as Rimadyl (carprofen),19 and most recently, joint regeneration (using fat derived stem cells) in dogs20 with degenerative hip disease are but a few examples of treatments developed via animal experimentation that now benefit countless companion animals around the world.
Research Drives Excellence
As to the utility of the canine TBW work, we were the first to demonstrate that intracellular solutions of the kind used for organ preservation were useful for prolonged asanguineous perfusion, the first to demonstrate that extended (4 hour) asanguineous perfusion was routinely survivable in dogs with normal mentation, and the first to demonstrate the conservation of mammalian memory and personality after cooling to ~5oC. The Society for Cryobiology refused to publish our paper, and they worked relentlessly to ensure that no other journal would publish a paper from ‘body freezers.’ This work is available on line, and it is now possible for people to judge for themselves. This work by Cryovita and Alcor, which was carried out in the early 1980s, was not repeated until 1991 with the publication of nearly identical work by Bailes, et al. Interestingly, one of the investigators on that paper (who later became the principal person conducting subsequent work in this area), was Michael Taylor, Ph.D., of the Society for Cryobiology, who also happened to be one of the reviewers who rejected the Cryovita-Alcor TBW study in the 1980s.20,21
As was the case in most animal research labs then, much of our equipment was ‘older but serviceable.’ Indeed, UCLA sometimes had equipment that was no better, and was not infrequently more time worn.
 Figure 20: Mike Darwin preparing an ‘ancient’ Travenol RSP dialysis machine for electrolyte normalization and hemoconcentration on the TBW dog ‘Enkidu’[2] on 17 March, 1984 at Cryovita Laboratories.
Figure 20: Mike Darwin preparing an ‘ancient’ Travenol RSP dialysis machine for electrolyte normalization and hemoconcentration on the TBW dog ‘Enkidu’[2] on 17 March, 1984 at Cryovita Laboratories.
Paradoxically, sometimes the latest equipment is an absolute barrier to its use in a research application by virtue of its technological sophistication. I used hemodialysis with ultrafiltration to normalize blood electrolytes in the animals after blood replacement and to extract water from the extracorporeal circuit and vascular space – the latter to raise both the oncotic pressure and the hematocrit – this was before the advent of microporous hollow fiber hemoconcentrators in CPB. Because the intracellular perfusate we used (MHP-2[3]) contained 40 mEq/L of potassium, and very little sodium, even after the blood was replaced, the animals had cardioplegic levels of potassium present in their blood. It was most efficient to normalize the blood potassium level via dialysis – but this required that the dialysate be customized to our application.
 Figure 21: Continuous monitoring of dialysate pH during rewarming with dynamic adjustment of pH being carried out by the addition of sodium hydroxide solution to the dialysate via the top ‘mixing compartment’ of the RSP dialysis machine. The bottle of Hemastix was used to check the dialysate returning from the dialyzer for hollow fiber breaks that could result in blood leaks from extracorporeal circuit into the dialysate.
Figure 21: Continuous monitoring of dialysate pH during rewarming with dynamic adjustment of pH being carried out by the addition of sodium hydroxide solution to the dialysate via the top ‘mixing compartment’ of the RSP dialysis machine. The bottle of Hemastix was used to check the dialysate returning from the dialyzer for hollow fiber breaks that could result in blood leaks from extracorporeal circuit into the dialysate.
Unfortunately, all of the then state-of-the-art equipment, such as the Cobe Sentry II dialysis machine, used ‘proportioning technology’ to mix the dialysate in real time; and these machines also had built in conductivity monitors that would shut the system down in the event the electrolyte concentration was sensed to be inadequate. It was also impossible to dynamically adjust the pH of the dialysate in a proportioning system. The very technological safeguards and ‘undefeatable’ automation built into the system to make it safe and more ‘foolproof’ also rendered it useless for research! As a consequence, I had to locate batch-type machines from the late 1960s, and single pass convertors from the 1980s, to allow for the use of hollow fiber dialyzers in order to use this modality on our animals. Newer isn’t always better.
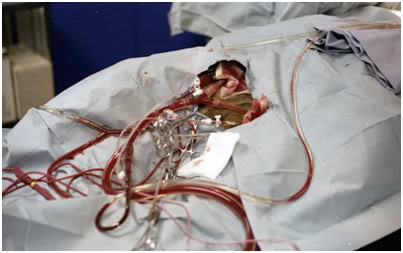 Figure 22: Bilateral femoral cannulation was used for the TBW experiments. In this photograph the arterial, venous, dialysis and pressure monitoring lines are visible during the perfusion of TBW dog ‘Enkidu’ on 17 March, 1984 at Cryovita Laboratories.
Figure 22: Bilateral femoral cannulation was used for the TBW experiments. In this photograph the arterial, venous, dialysis and pressure monitoring lines are visible during the perfusion of TBW dog ‘Enkidu’ on 17 March, 1984 at Cryovita Laboratories.
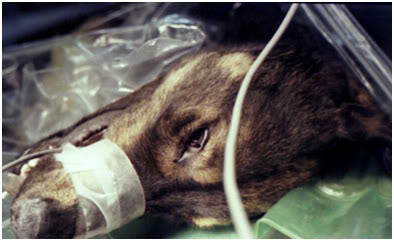
Figure 23: One unexpected consequence of using hyperosmolar extracellular solution for asanguineous perfusion was the extraction of non-vascular water from the aqueous and vitreous humors or the eyes (as well as the cerebrospinal fluid). As can be seen in this photo (TBW dog ‘Enkidu’ on 17 March, 1984 at Cryovita Laboratories), the eyes become sunken and flaccid during perfusion and this raised concern that retinal detachment might occur. This did not happen, and despite large reductions in brain volume and ‘ocular flattening,’ the animals recovered normally and lead normal, healthy lives, dying of old age in their mid-teens.
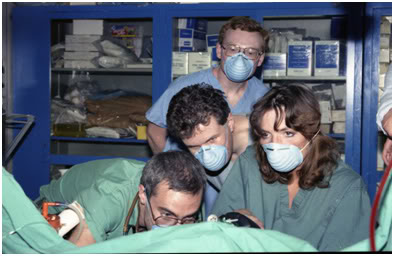 Figure 24: The Awakening: This candid photo perfectly captures the sense of wonder and anticipation that everyone who was involved in these experiments experienced at the time. When this photo was taken, Enkidu had just begun to recover a ‘lash reflex,’ indicating the imminent return of consciousness. From left to right: Mike Darwin, Garret Smyth, Max More and Brenda Peters.
Figure 24: The Awakening: This candid photo perfectly captures the sense of wonder and anticipation that everyone who was involved in these experiments experienced at the time. When this photo was taken, Enkidu had just begun to recover a ‘lash reflex,’ indicating the imminent return of consciousness. From left to right: Mike Darwin, Garret Smyth, Max More and Brenda Peters.
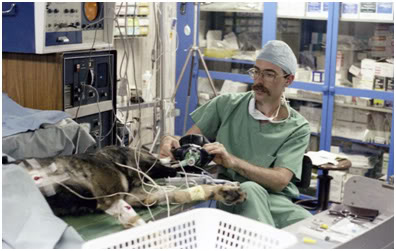 Figure 25: Mike Darwin giving ‘assist’ ventilations to a dog recovering from 4 hours of asanguineous perfusion at Cryovita Laboratories in 1981.
Figure 25: Mike Darwin giving ‘assist’ ventilations to a dog recovering from 4 hours of asanguineous perfusion at Cryovita Laboratories in 1981.
 Figure 26: Alcor member and volunteer Anna Tyeb restrains a rambunctious ‘Enkidu’ a few days after he underwent 4 hours of asanguineous perfusion at ~5oC.
Figure 26: Alcor member and volunteer Anna Tyeb restrains a rambunctious ‘Enkidu’ a few days after he underwent 4 hours of asanguineous perfusion at ~5oC.
There are literally hundreds of photographs of these experiments, as well as half a dozen videotapes; most of which have not yet been digitized. They document virtually every facet of the extracorporeal technology used to carry out this research. I do not merely believe, I know (from experience) that the equipment and techniques we employed were exemplary and representative of the standard of care in both experimental cardiopulmonary bypass laboratories, as well as many of the clinics of the day. Alcor cryonicists have a great deal to be proud of in terms of both advances in research, and clinical (cryopatient) care, during this period and throughout the 1980s (certainly up until the departure of Jerry Leaf and me from Alcor in 1991).
Why was this research done? For many reasons, not the least of which was that we hoped that by applying the same technology of intracellular perfusates that had allowed for 12 to 24 hour clinical cold storage of organs for transplant (such as the kidney and liver) to whole animals, we might possibly enable the creation of emergency ultraprofound ‘preservative hypothermia’ as a viable clinical modality. This technology would allow for rapid, in-field, or in-hospital asanguineous perfusion and cooling to a few degrees above 0oC; thus enabling victims of both civilian and battlefield exsanguinating trauma to enter a state of temporary ‘suspended animation,’ buying them time to reach sophisticated medical facilities where definitive treatment would be available. Twenty years later, this idea is now being seriously explored by some of the most outstanding research and clinical institutions in the US. Note the dates of publication on these papers – they come nearly two decades after we first successfully recovered dogs from four hours of asanguineous ultraprofound hypothermia:
1: Bellamy R, Safar P, Tisherman SA, Basford R, Bruttig SP, Capone A, Dubick MA, Ernster L, Hattler BG Jr, Hochachka P, Klain M, Kochanek PM, Kofke WA, Lancaster JR, McGowan FX Jr, Oeltgen PR, Severinghaus JW, Taylor MJ, Zar H. Suspended animation for delayed resuscitation. Crit Care Med. 1996 Feb;24(2 Suppl):S24-47. Review. PubMed PMID: 8608704.
2: Safar P, Tisherman SA, Behringer W, Capone A, Prueckner S, Radovsky A, Stezoski WS, Woods RJ. Suspended animation for delayed resuscitation from prolonged cardiac arrest that is unresuscitable by standard cardiopulmonary-cerebral resuscitation. Crit Care Med. 2000 Nov;28(11 Suppl):N214-8. Review. PubMed PMID: 11098950.
3: Tisherman SA. Suspended animation for resuscitation from exsanguinating hemorrhage. Crit Care Med. 2004 Feb;32(2 Suppl):S46-50. Review. PubMed PMID: 15043228.
4: Safar P, Behringer W, Böttiger BW, Sterz F. Cerebral resuscitation potentials for cardiac arrest. Crit Care Med. 2002 Apr;30(4 Suppl):S140-4. Review. PubMed/ PMID: 11940789.
5: Tisherman SA, Rodriguez A, Safar P. Therapeutic hypothermia in traumatology. Surg Clin North Am. 1999 Dec;79(6):1269-89. Review. PubMed PMID: 10625978
6: Marion DW, Leonov Y, Ginsberg M, Katz LM, Kochanek PM, Lechleuthner A, NemotoEM, Obrist W, Safar P, Sterz F, Tisherman SA, White RJ, Xiao F, Zar H.Resuscitative hypothermia. Crit Care Med. 1996 Feb;24(2 Suppl):S81-9. Review. PubMed PMID: 8608709.
7: Shoemaker WC, Peitzman AB, Bellamy R, Bellomo R, Bruttig SP, Capone A, DubickM, Kramer GC, McKenzie JE, Pepe PE, Safar P, Schlichtig R, Severinghaus JW, Tisherman SA, Wiklund L. Resuscitation from severe hemorrhage. Crit Care Med.1996 Feb;24(2 Suppl):S12-23. Review. PubMed PMID: 8608703.
8: Alam HB, Koustova E, Rhee P. Combat casualty care research: from bench to the battlefield. World J Surg. 2005;29 Suppl 1:S7-11. Review. PubMed PMID: 15815839.
9: Fukudome EY, Alam HB. Hypothermia in multisystem trauma. Crit Care Med. 2009 Jul;37(7 Suppl):S265-72. Review. PubMed PMID: 19535957.
10: Janata A, Weihs W, Schratter A, Bayegan K, Holzer M, Frossard M, Sipos W, Springler G, Schmidt P, Sterz F, Losert UM, Laggner AN, Kochanek PM, Behringer W. Cold aortic flush and chest compressions enable good neurologic outcome after 15 mins of ventricular fibrillation in cardiac arrest in pigs. Crit Care Med. 2010;38(8):1637-43. PubMed PMID: 20543671.
End of Part 3
References
12) Leaf, JD. Federowicz, M. Hixon, H. Case report: two consecutive suspensions, a comparative study in experimental human suspended animation. Cryonics. 6(11):13-38;1985: http://www.alcor.org/Library/html/casereport8511.html. Retrieved 2010-08-31.
13) Darwin, M, Interview with Jerry Leaf, Part I. Cryonics. 7(7);26-34:1986. http://www.alcor.org/Library/html/Interview-JerryLeaf.html. Retrieved 2011-01-24.
14) Leaf, JD, Darwin, M, Hixon, H. A mannitol-based perfusate for reversible 5-hour asanguineous ultraprofound hypothermia in canines: http://www.alcor.org/Library/html/tbwcanine.html. Retrieved 2011-02-24.
15) Darwin, M. The myth of the golden scalpel. Cryonics. 7(1);15-18:1986: http://www.alcor.org/Library/html/MythOfTheGoldenScalpel.html. Retrieved 2011-01-24.
16) Darwin, M, Interview with Jerry Leaf, Part I. Cryonics. 7(7);26-34:1986. http://www.alcor.org/Library/html/Interview-JerryLeaf.html. Retrieved 2011-01-24.
17) http://www.banpoundseizure.org/yourstate.shtml. Retrieved 2011-01-24.
18) PUBLIC LAW 101-624–NOV. 28, Protection of Pets. 1990http://awic.nal.usda.gov/nal_display/index.php?info_center=3%20&tax_level=4&tax_subject=182&topic_id=1118&level3_id=6735&level4_id=11096&level5_id=0&placement_default=0. Retrieved 2011-01-25.
19) Committee for the Evaluation of Veterinary Products, European Agency for the Evaluation of Medicinal Products, EMEA/MRL/042/95/FINAL. http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500011412.pdf. Retrieved 2011-01-25.
20) http://www.vet-stem.com/smallanimal/. Retrieved 2011-01-24.
21) Bailes JE, Leavitt ML, Teeple E, Maroon JC, Shih SR, Marquart M, Elrifai AM, Manack L. Ultraprofound hypothermia with complete blood substitution in a canine model. J Neurosurg. 1991;74:781-788.
22) Elrifai AM, Bailes JE, Leavitt ML, Teeple E, Shih SR, Taylor MJ, Maroon JC, Ciongoli KA, Devenyi C, Rosenberg I. Blood substitution: an experimental study. J Extracorp Tech. 1992;24:58-63.
[1] The United States Department of Agriculture is the regulatory agency that oversees animal research facilities in the US.
[2] The name for this dog was chosen by Alcor activist and research volunteer Anna Tyeb. Enkidu was a character in the Sumerian Epic of Gilgamesh, the oldest known human book, recorded on 12 clay tablets dated to ~3000 BCE. Enkidu is a wild-man who symbolizes the untamed natural world, and though he is the cultured Gilgamesh’s equal in strength and courage, he is in many ways his antithesis. After a wrestling match between the two, Enkidu becomes a friend and soul mate, and Gilgamesh’s constant companion in adventure; until he is taken ill and dies. The death of Enkidu wounds and enrages Gilgamesh, forcing him to realize that he had “loved and lost a friend to death, and learned he lacked the power to bring him back to life.” This inspires Gilgamesh on a quest to escape death by obtaining physical immortality.
[3] MHP is Mannitol-HEPES perfusate; mannitol is the impermeant sugar-alcohol and the zwitterionic buffer sodium HEPES was the primary buffer.

Even the Congressional Budget Office and the Social Security trustees appointed by the president say that Social Security is financially sound, without any changes for the next 40 to 50 years.
Surely you jest? — Mike Darwin